On-Time Trials Annual Update
We have had a busy start to 2023. Over the last few months, the team at On-Time Trials has been hard at work to capitalize on the success we had with the Lighthouse accelerator program. At some point in the past, we have crossed paths and I would like to update you on some of the progress that we have made during this time. As we have been moving quickly, we also want to improve our communication cadence with monthly investor updates that include progress reports celebrating any wins, Key Performance Indicators (KPI’s), any potential roadblocks, and help requests to our community. This 2023 update will be longer than the monthly cadence so that we can share all of our exciting new.
OTT Overview
If you are in need of a refresher, my name is Trevor Coughlan, founder and CEO of On-Time Trials (OTT), a SaaS-based clinical trial optimization software company. We help clinical trial sponsors (Drug & Device Developers) utilize existing data to become more efficient and effective at planning and completing clinical trials on-budget and on-time, early or to fail fast when needed.
The short value prop in a few words is as follows:
– Imagine you could scale and execute clinical trials to finish on time or early with accuracy, repeatability and high confidence. This would enable early revenue & margin capture and more effective pipeline management (with the ability to fail fast & redeploy resources) for Sponsors, CROs, Sites and Patients. On-Time Trials (OntimeTrials.com) has the solution to unlock this transformative value. Please explore this video on OTT’s home page and we look forward to discussions!

FUNDRAISING $1.5M SEED ROUND
We are actively working with the investor community to raise $1.5-2M to fund our active pipeline. We are seeking strategic, connected investors to help fund our go-to-market strategy which includes building out our sales function along with conference exhibits and sponsorship to promote the OTT Brand and Software. Thus far, OTT has bootstrapped the development of enterprise-level software that is ready for deployment. However, we now need to showcase the potential cost savings through an experienced sales team, including customer travel and event showcases. This investment will give us 14 months of runway to sell and onboard the 3-4 new mid-sized clients that will push OTT to cash flow positive. We are using a new software, FINTA, to manage the fundraising process and investment documents and the deal room can be found at the following link: https://app.trustfinta.com/i/on-time-trials/BfQT75z-e
Team Members & Advisors
Since winning the Lighthouse Labs accelerator competition, we have added several advisors and part-time employees.
Founders

Trevor Coughlan
CEO – Co Founder
28 years operations leader with Hi-Tech Manufacturing &, clinical operations. Designed the OTT Platform after a successful pilot. Master Black Belt in Lean systems and Technology.

Kishore Manoharan
CTO – Co Founder
20+ years Highly skilled Solution Architect. Built the On-Time Trials Platform that is ready to use.

Nikhil Parekh
Innovation Advisor
An enterprise and a visionary leader with 35+ years of experience in discovery, development, in-licensing and commercialization of innovative consumer health care and prescription pharmaceutical products.

Tim Van Meter
Medical Advisor

Eric Hamborg
Commercial Advisor
25+ years in Health care Sales in Pharma and former co founder of a health care services company

Sean McCluskey
Strategic Advisor
28 years Leadership in US Health Care / Si Valley, Payers & Disruptive Innovators.

Stu Schulman
Commercial Advisor

Kevin Futch
Finance & Operations Advisor
20+ years of finance/controllership across multiple industries. Co-founder/CFO for software startup and graduate of GE Financial Leadership program with an MBA from the University of Southern California.
FIRST CUSTOMERS AND IMPACT OF OTT SOFTWARE
Since graduating from Lighthouse Labs, OTT has been working to meet as many potential customers as possible to communicate the benefits from OTT Software. We have had a number of meetings, in-person and zoom, with Sponsors and CRO’s of all sizes. The feedback has been excellent thus far and we have been working closely with our first customer since the middle of October.
Client Overview
Sponsor of a device trial that had hired a 3rd party CRO to manage their trial over 11 sites. Since onboarding OTT, the sponsor has made the following progress:
- BEFORE OTT: Client was using EDC and multiple spreadsheets to manage the trial and sites. Sponsor PM was very frustrated with CRO and overall site performance. Lack of visibility made it unclear why the trial was behind schedule. Trial KPI’s were very poor.
- ONBOARDING: Client onboarded to OTT software in less than a week. Initial output showed all sites behind schedule and data page verification at 95%.
OTT Impact
In the 13 weeks since installing OTT the Sponsor has made the following progress:
- Increased enrollment rate per day from 0.84 to 2.2 (>160% increase)
- Increased data pages entered /day from 5.8 to 30.3 (>400% increase)
- Increased data pages verified / day from 1.8 to 32.39 (1700% increase)
- Sponsor has reduced dependency on the CRO and brought in their own CRA
- Utilizing motion charts to provide transparency across all sites and functions, increasing productivity at underperforming sites and to more closely manage the CRO
- Based on results from OTT Resource Module, determined that contract with CRO is over-resourced and not cost-effective
Results:
Initial OTT contract was a trade-out with a deliverable of a joint whitepaper along with business development testimonial. Client has committed to using OTT going forward utilizing active site-based pricing.
Product Enhancement and Roadmap
In addition to onboarding new clients, we have been busy utilizing feedback to build a more robust product offering. While the software is ready for enterprise-level deployment, adding new scalability and functionality is at the core of our product offering. Below are several enhancements as well as updates to the product roadmap:
Product Enhancement:
- Implementation of Social Determination of Health (SDoH) – Refining method for tracking recruitment in motion charts
- Development of Training Matrix – includes a 360 degree review of CRA’s on operational efficiency. Measures level of proficiency in 3 factors: screening, completing study, and follow up. Low performance or new employees trigger allocation of more time on site.
- Project Management & task management tools broader availability and usability of tasks that trigger alerts and can be assigned to specific users and tracked to closure.
Product Roadmap:
- Enhanced patient screening – identify and develop partnership with EDC and Decision-Based Criteria software to complement and build out the full suite of features.
INDUSTRY NEWS AND UPDATES
Major drug manufacturers, coming off of record financial performance in 2022, are looking for ways to increase profit and expedite their pipeline through the approval process to backfill revenue. OTT provides visibility throughout the pipeline to Sponsors, allowing studies to stay on-time or finish early. It also allows Sponsors to fail drugs 30-50% earlier to free up resources for other opportunities in the pipeline.
- Pfizer Facing Profit Drop After Record Year as COVID Vaccine Demand Fades
- Market gears up for biosimilar boom in 2023 as Humira exclusivity draws to a close
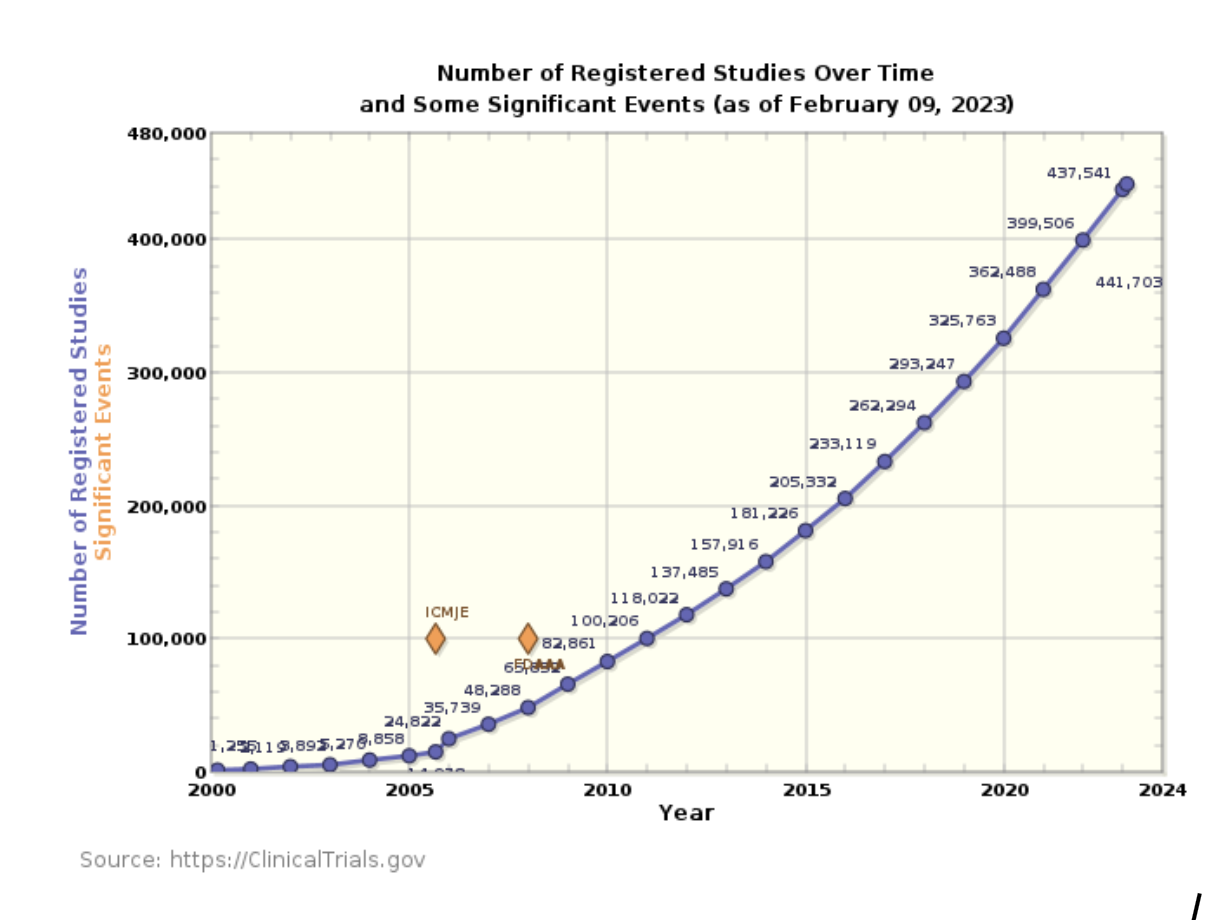
CRA’s (clinical research associates) are facing increased pressure due to the number of studies they are managing, the additional complexity, and the expected timeline to complete the trials. This is driving significant turnover in the profession and making it difficult to staff the number of registered studies.
The number of newly registered studies grew by 10% in 2023 and is on pace to exceed that growth in 2023. The current number of active registered studies is 442,235. Over 90% of these studies will be delayed, costing Sponsors millions of dollars for each day of delay. Of these studies, roughly one third are in Phase 2 or Phase 3. These studies can cost hundreds of millions of dollars to complete and are the most realistic opportunity of sell-through for OTT software.
I hope your year is off to a great start. If you would like to learn more or connect directly, you can use the Calendly link to book 30 minutes on my calendar. In addition, if you would not like to receive our monthly updates, you can unsubscribe at any time. I look forward to hearing from you.
Best
Trevor Coughlan
CEO – On-Time Trials
https://calendly.com/tcoughlan-ontimetrials/30min
Are you interested in helping On-Time Trials to accelerate drug and device development ?
